The Big Picture for the Science of Consciousness
by Ravi Khanna
“Science & Spirituality in Modern India”
Feb. 5 – 7, 2006
The Big Picture for the Science of Consciousness
INTRODUCTION
The western study of consciousness has progressed very rapidly in the last century but it is still stuck in the mould of mind – body dualism. This is best brought out in the words of a recent Time magazine quote [1]….
“If you close your eyes and think about it for a while, as philosophers have done for centuries, the world of the mind seems very different from the one inhabited by our bodies. The psychic space inside our heads is infinite and ethereal; it seems obvious that it must be made of different stuff than all the other organs. Cut into the body, and blood pours forth. But slice into the brain, and thoughts and emotions don’t spill out onto the operating table. Love and anger can’t be collected in a test tube to be weighed and measured.
René Descartes, the great 17th century French mathematician and philosopher, enshrined this metaphysical divide in what came to be known in Western philosophy as mind-body dualism. Many Eastern mystical traditions, contemplating the same inner space, have come to the opposite conclusion. They teach that the mind and body belong to an indivisible continuum.”
What constitutes this ‘indivisible continuum’ ? We are going to look at this in terms of the Vedas in this paper. As we will see, this leads us to the larger picture of consciousness as it is presented in the east and we will compare this with the western contemporary concepts of Multiple Universes or Multiverses and the theory of Infinities. Both these western scientific developments that have emerged in the last century also push the mind towards the ‘Big picture’.
IS THE MIND AN ‘EMERGENT’ PROPERTY OF MATTER ?
William James (1842 – 1910), the father of American Psychology observed that Consciousness is not a ‘thing’ but a ‘process’. In the last century the western study of neurobiology, its chemical processes and imaging techniques have undergone phenomenal advances carrying this ‘study of processes’ to cognitive sciences. The brain was studied using sophisticated EEG (Electro Encelograph) and MRI (Magnetic Resonance Imaging) machines to map the visual cortex, language areas, auditory lobes, the relation of fear to amygdala and so on and so forth [2]. But where exactly does the Consciousness reside in the cerebellum ? …. in the hippocampus or cerebrum – there are no easy answers !
So we come to what David J. Chalmers calls the ‘HARD PROBLEM’ [3] …. “ The Hard Problem is the question of how physical processes in the brain give rise to subjective experience. This puzzle involves the inner aspect of thought and perception : the way things feel for the subject. When we see, for example, we experience visual sensations, such as that of vivid blue. Or think of the ineffable sound of a distant oboe, the agony of intense pain, the sparkle of happiness or the meditative quality of a moment lost in thought. All are part of what I call consciousness. It is these phenomena that pose the real mystery of the mind.” In a serious attempt to address this ‘Hard Problem’ His Holiness the Dalai Lama has been holding a series of Mind & Life talks with eminent western scientists every two years since October 1987 [4]. The challenge has been to trace the advent of consciousness into the human mind from the progression of matter and its evolution. Some of the interesting features of these and other related writings are as follows:-
In a serious attempt to address this ‘Hard Problem’ His Holiness the Dalai Lama has been holding a series of Mind & Life talks with eminent western scientists every two years since October 1987 [4]. The challenge has been to trace the advent of consciousness into the human mind from the progression of matter and its evolution. Some of the interesting features of these and other related writings are as follows:-
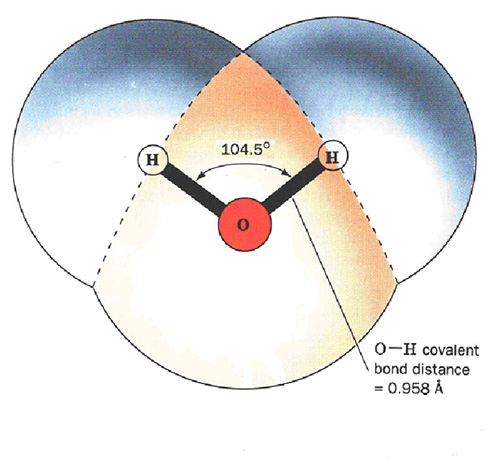
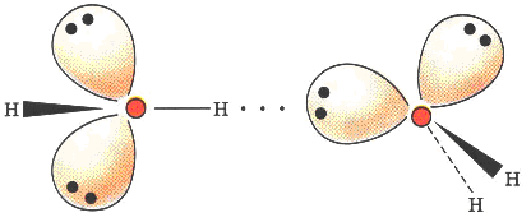
- The Water Molecule The first remarkable substance is ‘Water’. It is made up one oxygen atom and two hydrogen atoms which bond in a ‘covalent’ manner to give the water molecule an internal ‘mickey mouse’ symmetry.[5] The 104.5 ° angle is not sacrosanct and varies as external electrostatic forces apply. This geometry also gives an electric dipole moment to the water molecule that accounts for water’s remarkable ability to dissolve a large variety of substances whose molecules are electrically bounded together. This is an ‘ionic’ bond and is external. It is the combination of this dual bonding capability that positions water as the unique ‘life – force’ on our planet. For example it co-exists as water-vapor, liquid and ice simultaneously at earth temperatures and is also the densest at 4 °C. Thus, whereas all other solids are heavier than their liquid states , ‘ice’ floats on water. It is this property that has preserved life through the ‘ice-ages’ where even though the surface of the oceans were frozen the bottom was always warm enough to support continuity.
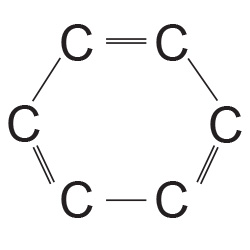
- The Carbon Ring of Benzene The second amazing fact is the structure of the carbon atom. It has an interesting symmetry of ‘four’ valence electrons. It is this that allows carbon to crystallize in tight, tetrahedral structures of diamond. Carbon can bond in a ‘linear’ fashion to create long chains of organic compounds or it can also form ‘circular’ closed benzene-like structures with the symmetry of six atoms.
 In this manner carbon combined with an abundant supply of hydrogen, nitrogen, oxygen, phosphorus and sulfur gives rise to long chains of organic chemicals which in turn are the very basis of the life-giving complex chemicals.[6]
In this manner carbon combined with an abundant supply of hydrogen, nitrogen, oxygen, phosphorus and sulfur gives rise to long chains of organic chemicals which in turn are the very basis of the life-giving complex chemicals.[6]
3 Billion Years ago the earth’s condition was that of extreme temperature and pressure and molten lava was hissing in the steam of water pools amidst lightening strikes. The conditions were perfect for all these unique properties of water and carbon to combine with other elements into a chemical soup from which emerged life sustaining chemical structures like amino acids, proteins, enzymes and other carbon compounds.[7] An interesting point in the study of the Benzene ring is that each carbon atom has three bonds with its two neighbours in the circle – the fourth extends radially outwards and is available for bonding with other atoms e.g. hydrogen, nitrogen, oxygen or another carbon etc.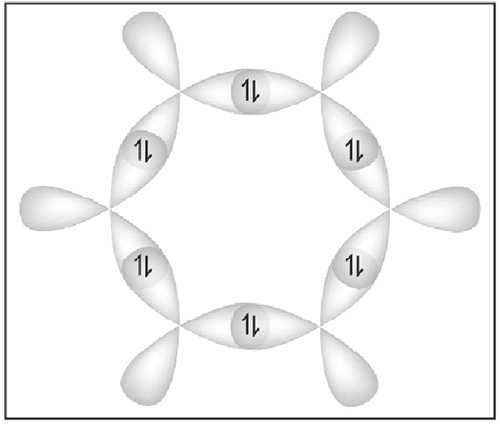 The diagram[8] on the side shows two of these bonds with each neighbour and these are like six children holding hands in a circular game. These bonds are spatially restricted and are called ‘s – bonds’ (sigma – bonds). The third bond is vertical to this plane (going in & out of the paper) and is called the ‘p – bond’ (pi – bond). The six ‘p – bonds’ merge into each other and they are not spatially restricted over the entire circular region.Thus the ‘s – bonds’ are the backbone of the chemical structure and unfold the spatial dimension of the nucleic acids, proteins etc. On the other hand, the ‘p – bonds’ merge together in delocalized geometries and they can be said to have an extra degree of freedom. Remember that the shapes shown of these ‘bonds’ are probability distributions of occurrence of electrons in two phases or ‘spins’.
The diagram[8] on the side shows two of these bonds with each neighbour and these are like six children holding hands in a circular game. These bonds are spatially restricted and are called ‘s – bonds’ (sigma – bonds). The third bond is vertical to this plane (going in & out of the paper) and is called the ‘p – bond’ (pi – bond). The six ‘p – bonds’ merge into each other and they are not spatially restricted over the entire circular region.Thus the ‘s – bonds’ are the backbone of the chemical structure and unfold the spatial dimension of the nucleic acids, proteins etc. On the other hand, the ‘p – bonds’ merge together in delocalized geometries and they can be said to have an extra degree of freedom. Remember that the shapes shown of these ‘bonds’ are probability distributions of occurrence of electrons in two phases or ‘spins’.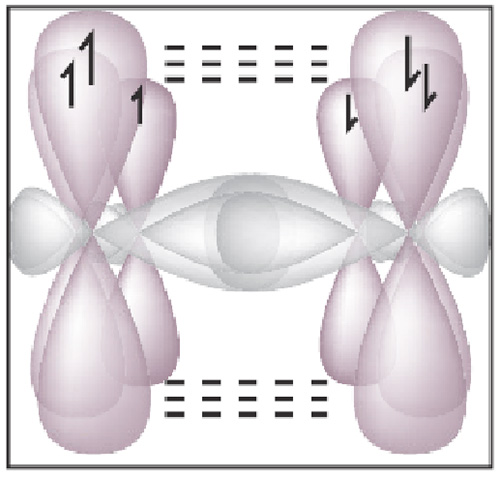
- The Protocell – Lipid Vesicles The next crucial step in pre-biotic evolution, 3 Billion years back, seems to be the formation of the simple cell. This combines the first two facts of both water and carbon cited above. An interesting description of this has been given by Fritjof Capra[9]:-
‘It is the closure of a primitive membrane into a “vesicle” that represents a discrete transition from non-life to life.’ (Harold Morowitz)
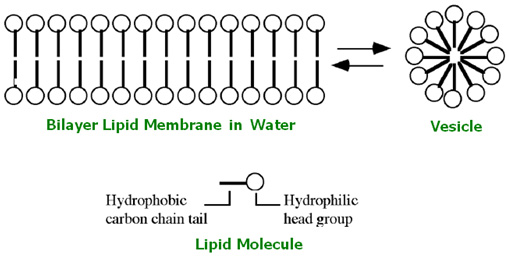
The chemistry of this crucial process is surprisingly simple and common. It is based on the electric polarity of water. Because of this polarity, certain molecules are hydrophilic (attracted by water), while others are hydrophobic (repelled by water). A third kind of molecules are those of fatty and oily substances, known as lipids. They are elongated carbon structures with one hydrophilic and one hydrophobic end. (See Picture)
When these lipids come in contact with water, they spontaneously form various structures. For example, they may form a mono-molecular film spreading over the water surface or alternatively they may form an even more complex double layer of molecules with water on both sides, as shown in the Picture. These single or double layers can form droplets, which are the membrane-bounded vesicles. These double-layered greasy membranes show a surprising number of properties similar to contemporary cellular membranes. They restrict the number of molecules that can enter the vesicle, transform solar energy into electrical energy and even collect phosphate compounds inside their structure.
Lipid vesicles, then, are the ideal candidates for the protocells out of which the living cells evolved.
This was the first semi-permeable boundary between the “outside” and the “inside”; the first distinction between self and non-self.[10]
- Dissipative Structures and Autopoietic Organization The complexity of the bio-chemical process took another 200 million years or so to progress from the protocell to the prokaryote, a single living cell.
The protocells were the hotbed of a lot of molecular activity. Complex chains of linear polymers of carbon compounds gave rise to life-building amino acids, nucleotide and simple sugars. As the molecules became bigger their chemical reactions became ‘auto catalytic’ and cyclic i.e. the derivatives promoted further generation of themselves and related products. These hyper-cycles were possible due to accumulation of energy within the first cells and more complex protein, DNA and RNA structures arose. This is the very point at which non-sentient matter gives ‘life’ to sentient cell structures. The energy accumulation inside spherical protocells pushed the chemical reactions to ‘far from equilibrium’ levels of energy, thereby creating more complex structures called “dissipative structures” which have self-organizational capability. If we look at this in terms of systems development then, this higher level of self-generating capability is called ‘an autopoietic organization’:-
- The first cell-like systems were what the Belgian Nobel Prize – winning physicist Ilya Prigogine has termed “dissipative structures” – objects or processes that organize themselves and spontaneously change their form. With an influx of energy, dissipative structures may become more instead of less ordered….In dissipative structures, information begins to organize itself; pockets of elaboration arise.
From dissipative structures and hypercycles emerged the chain of nucleotides, ribose, and phosphate that can both replicate itself and catalyze chemical reactions.[11]
- How do I know when a being is living? Throughout the history of biology many criteria have been proposed. They all have drawbacks. ….When we speak of living beings, we presuppose something in common between them; ….Our proposition is that living beings are characterized in that, literally they are continually self-producing. We indicate this process when we call the organization that defines them an autopoietic organization.
First, the molecular components of a cellular autopoietic unity must be dynamically related in a network of ongoing interactions. Today we know many of the specific chemical transformations in this network, and the biochemist collectively terms them “cell metabolism”. Interestingly, this cell metabolism produces components which make up the network of transformations that produced them. Some of these components form a boundary, a limit to this network of transformations. In morphologic terms, the structure that makes this cleavage in space possible is called a membrane. (Underlining by me) ….. What we have, then, is a unique situation as regards relations of chemical transformations: on the one hand, we see a network of dynamic transformations that produces its own components and that is essential for a boundary; on the other hand, we see a boundary that is essential for the operation of the network of transformations which produced it as a unity:…… The most striking feature of an autopoietic system is that it pulls itself by its own bootstraps and becomes distinct from its environment through its own dynamics, in such a way that both things are inseparable. Living beings are characterized by their autopoietic organization. They differ from each other in their structure, but they are alike in their organization. [12]
- Hypercycles – trap large energy and release in small steps There are thousands of these autocatalytic hypercycles of chemical reactions within the cell and their bio-chemistry is far from being completely understood[13]. A salient feature of these metabolic processes is that they capture high-energy electrons and release the energy in small steps that are conducive for daily biological needs. Photosynthesis is one of these processes in which high energy photons of light are trapped – an emergent phenomena in the evolution of life:-
- The machinery of cells, which is assembled in a variety of short and long-chain carbon compounds, composed of elements compounded from inorganic matter, is centrally important here as it consists of self-organizing emergent phenomena. By this time at least, the controlled cascade of electron energy, the earliest beginnings of metabolic machinery has emerged. Photosynthesis, the process of capturing high-energy electrons to make sugars and amino acids out of carbon dioxide and water and nitrogen, is a self-renewing source of high-to-low energy electrons. Ways were “found”, or selected, to take electron energies – in ordered sequences and branching pathways – from high to low energy levels in small steps. The essential process was the controlled reduction of electron energy in a staircase of small steps, as in a flowing cascade of water, rather than in an abrupt fall as in a waterfall. The controlled energy decrements are utilized to assemble molecules, to transport substances within cells, to transport products across cell membranes, as in secretion and neurotransmission, to provide motile power to cells, to contract muscles, to control the dance of chromosomes and other aspects of cell division, to control the fusion of cells, as in fertilization, and to operate all the innumerable physiological systems in multicellular organisms.
This controlled electron energy decrement is something not seen in the organic world. Something radically new has been added to the universe in this process. The kinds of abrupt shifts in the inorganic world like ionizing radiation, radioactivity etc. is actually destructive to living systems.[14]
- With porphyrin ring production in their repertoire, many types of bacteria evolved the ability to use the most reliable and abundant source of energy around: sunlight. When any molecule absorbs light, its electrons are boosted to a higher energy state. Usually the energy is simply dissipated as light or heat until the molecule returns to its normal state. But when the molecules are bound to porphyrins attached to proteins embedded in membranes as electron-transport chains, light energy can be put to use……This process of getting food from light and air – photosynthesis- freed some kinds of bacteria from their dependence on pre-formed organic compounds.
The evolution of photosynthesis is undoubtedly the most important single metabolic innovation in the history of life on the planet. It occurred not in plants, but in bacteria.[15]
- Microtubules – motility, mitosis The simple prokaryotic cell over the next few hundred million years diversified into various forms of bacteria that entered symbiotic mergers to give birth to the nucleated eukaryotic cell. This was the precursor to the modern day animal cells with a nucleus housing the chromosomes, the mitochondria for energy related processes, the ribosomes for protein formation, plastids and many other organelles. Housing a more evolved biochemistry the eukaryotic cells were better equipped to adapt to the climatic changes and competition for food. They thus developed, many survival structures and one of the major breakthroughs was motility – the ability to move. This ability in the simple prokaryotic appeared as a flagellum, a tail-like ‘whip’, and certain spiral-looking bacterium called ‘spirochetes’ developed this capability and attached themselves to other host bacteria helping to move them around. This ‘conscious mobility’ was a remarkable ‘push’ to the evolutionary process not only in the external sense but, as we will see below, also in terms of the seed for both reproduction and nervous development of subsequent life.
- In the single cell paramecium for example these whip-like cilia provide not only movement but are literally the cytoskeleton that supports the outer extreme structure. It is constituted of microtubules that are tiny bundles of protein fibers arranged in a (9 pairs + 2) structure that looks like a ‘telephone dial’. Besides holding the cell in shape these microtubules – “play a role for the single cell rather like a combination of skeleton, muscle system, legs, blood circulatory system and nervous system all rolled into one!” [16]
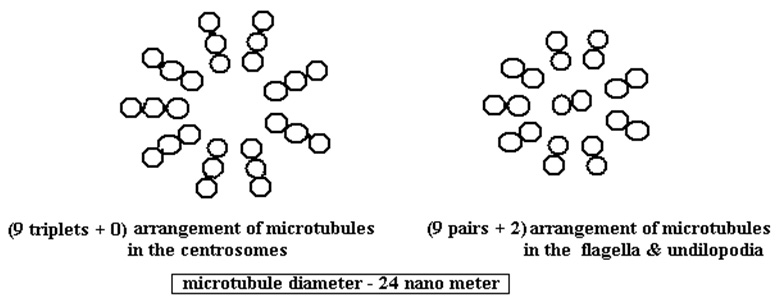
- A microtubular structure, that is random, is also found in the original spirochete bacterium and it is believed that these sphirochetes would attach themselves to other prokaryotes. This attachment to the host cells lead to the development of the whip-like tails, also called undulipodia, that assist in regular cell movement. But this is not all. The same tubules are found inside the eukaryotic cells and are responsible for their mitotic cell-division capability. They play an important role in the process of mitosis in which the chromosomes in the cell-nucleus duplicate themselves and subsequently the cell divides into two identical replicas. In words of Lynn Margulis – “But the intricacy of the mitotic dance becomes still more understandable if you allow for a spirochetal choreographer. And the clues are there. The spindle is made of microtubules, the same microtubules found in all cell undilopodia.”[17] The arrangement of the microtubules here is (9 triplets + 0).
- From here these microtubules have evolved to our brain cells and they frame the very structure of each neuron. But before we go further there is another very interesting phenomena relating to these microtubules. Either the microtubules are present in the whip or they are drawn inside by the cell for reproducing through the process of mitosis. In the words of Lynn Margulis – “In plants and animals, undilopodia and mitosis are mutually exclusive – they are never seen in the same cell at the same time. Fungal cells seem to have permanently traded cell whips for mitosis. But for some protists to divide, they must first pull their undilopodia inside their cells. No mammal cell – not to mention many other kinds of cells – can retain undilopodia while it divides by mitosis. It is as if the cell must use its ancient spirochetal symbionts for one thing or the other, but not both.”[18]
- In the single cell paramecium for example these whip-like cilia provide not only movement but are literally the cytoskeleton that supports the outer extreme structure. It is constituted of microtubules that are tiny bundles of protein fibers arranged in a (9 pairs + 2) structure that looks like a ‘telephone dial’. Besides holding the cell in shape these microtubules – “play a role for the single cell rather like a combination of skeleton, muscle system, legs, blood circulatory system and nervous system all rolled into one!” [16]
- Microtubules and the Mind The same microtubules in varying clusters are present in the axons and dendrites of the neurons in our brains (See Picture)[19].
 Roger Penrose has carried this discussion to the level of quantum coherence in the microtubules that provide the cytoskeleton to the axon and dendrites in the neuron.
Roger Penrose has carried this discussion to the level of quantum coherence in the microtubules that provide the cytoskeleton to the axon and dendrites in the neuron.
- What is the significance of microtubules for neurons? Each individual neuron has its own cytoskeleton. In particular, microtubules in neurons can be very long indeed….Moreover, they can grow or shrink, according to circumstances, and transport neurotransmitter molecules. There are microtubules running along the lengths of the axons and dendrites. Although single microtubules do not seem to extend individually to the entire length of an axon, they certainly form communicating networks that do so, each microtubule communicating with the next ones by means of microtubule associated proteins (MAPs). Microtubules seem to be responsible for maintaining the strengths of the synapses (nerve endings). Moreover, they seem to organize the growth of new nerve endings, guiding them towards their connections with other nerve cells.[20]
- The rod and cone nerve cells in the eye reveal the (9 pairs + 2) pattern of microtubules. The axons and dendrites of the brain are a differently organized mass of microtubules, containing all the microtubular proteins but without the (9 + 2) formation…. “Did the spirochete motility system of the microcosm evolve within the ordered environment of larger organisms to become the basis of their nervous systems?”…. After maturity brain cells never divide, nor do they move about. Yet we know mammal brain cells – the richest source of tubulin protein anywhere – do not waste their rich microtubular heritage. Rather, the sole function of mature brain cells, once reproduced or deployed, is to send signals and receive them, as if the microtubules once used for cell-whip and chromosomal movement had been usurped for the function of thought.[21]
It is very clear from the above excerpts that inorganic matter over the 3 billion years or so of evolution has carried the complexity of bio-chemistry through varying layers of complexity. From the merging of electron probabilities in the benzene ‘p – bonds’, to the coming together of bio-chemical ‘hypercycles, to the structure of the first simple cell – the prokaryote, to the emergence of the first single-cell eukaryote with the ‘whip’, to the multicellular mammals there are different dimensions of emergent phenomena. H.J. Morowitz[22] uses his expertise in Biology and Complexity to actually explain 28 steps of emergence for human evolution.
In terms of consciousness, the microtubules seem to play a pivotal role. They provide the first nervous impulse to dormant cells and are instrumental in their motility or ‘first movement’. At this point, they actually provide multiple functions to the single cell – ‘of skeleton, muscle system, legs, blood circulatory system and nervous system all rolled into one!’, as mentioned above. This is the point of sentient matter. The microtubule has the dimensions that are small enough and yet its structure is rigid enough to merge electron probabilities at the quantum level. This occurs not only along its tiny 24 nano-meter diameter but the confluence carries over its longer living length which is in milli-meters. The other interesting fact is the involution of the microtubules into some of the complex cells, the eukaryotes, to help them reproduce. This is the stage at which the complexity of life allows one structure to decay and another replica to replace its function. The microtubules are the very conduit that pull the DNA apart and help it duplicate. Again as, one becomes two, another sentient consciousness is birthed.
We have come to understand so far how the western ideas are sitting at the cutting edge of quantum ideas in an attempt to comprehend consciousness. But the study still has a reductionist flavor. We next turn to the east for solace. As the Dalai Lama exhorts us in his recent book[23] – “A neuroscientist maybe can tell us whether a subject is dreaming, but can a neurobiological account explain the content of a dream?
Assuming that the Mind is an emergent property of matter leaves a huge explanatory gap. How do we explain the emergence of Consciousness? What marks the transition from non-sentient to sentient beings? We must ‘emerge’ from the complexity of the descriptive process to understand the ‘mystery’ of Life.”
THE EASTERN MIND
The Vedic concept of consciousness does not dissect the brain and matter into its material constituents. It looks for the ‘spirit of the matter’. It treats the nervous system as ‘continuous’ with a ‘Higher consciousness’ ; the Unknown – this is the cidākāśa of Yoga Vasisṭha or the Brahmn of the Upanishads.
- The Yoga Vasistha and Consciousness The Yoga Vasistha of Vālmīki [24] dwells extensively on this subject and states in Section III – Utpatti Prakaranam, (Y.V III.17.10) the section dealing with creation :-
cittākāśam cidākāśam ākāśam ca trtīyakam,
dvābhyām śūnyataram viddhi cidākāśam varānane (10)
O Līlā, there are three types of space – the psychological space, the physical space, and the infinite space of consciousness. Of these the infinite space of consciousness is the most subtle and the other two find expanse in it.[25]
The word ‘space’ is not an adequate translation of the word ākāśh – it is more like “dimension”. There is an unfolding process of these dimensions by the chaitanya or our self-awareness. As the child grows it becomes aware of its cittākāśa, the psychological dimension and understands the limitation of its skin as the boundary between the ‘inside’ and the ‘outside’. The mind folds back and looks at the bhūtākāśa, the physical dimension that includes the elements in the external reality. The intellect discerns patterns, nomenclates them, learns linguistics and semiotic gestures thus building a repertoire of ‘higher’ awareness. Let us look at some later śhlökas explaining this (Y.V.III.97.16 & 17):-
sabāhya abhyanta atho yaḥ satta asatta avbodhakaḥ,
vyāpī samasta bhūtānāṁ cidākāśa sa ucyate (16)
sarva bhūthitaḥ shreṣṭho yaḥ kālkalnātmakaḥ,
yena admātataṁ sarvaṁ cittākāśaḥ sa ucyate (17)
The infinite space of undivided consciousness (cid ākāśa) is that which exists in all inside and outside, as the pure witness of that which is in substance or only as a vaporous intent. The finite space of divided consciousness (citta ākāśa) is that which creates the divisions of time, which pervades all beings and which has spread out the other spheres in immense vacuity.
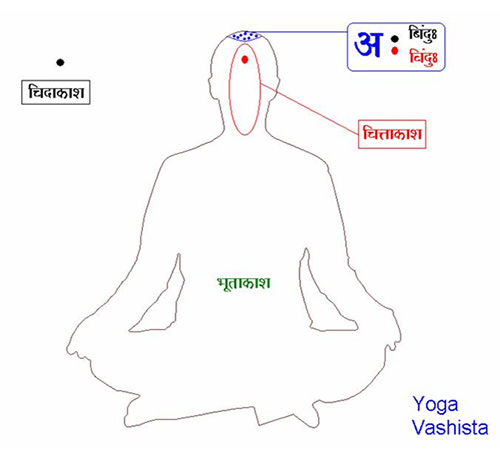 The text then explains that both the psychological and physical dimensions are subjugated to the infinite space of undivided consciousness – “In fact, the others do not exist, and this division of consciousness into three is arbitrarily suggested only while instructing the ignorant. The enlightened one knows that there is only one reality”.
The text then explains that both the psychological and physical dimensions are subjugated to the infinite space of undivided consciousness – “In fact, the others do not exist, and this division of consciousness into three is arbitrarily suggested only while instructing the ignorant. The enlightened one knows that there is only one reality”.
Thus the (cid ākāśa), the infinite space of undivided consciousness is the multidimensional field of Brahmn, the Unknown of the Vedas.
- The Aitareya Āraṇyaka
The Aitareya Āraṇyaka [26] is one of the oldest Vedic texts and at II.4.1 the Hymn of Creation commences which is also the Upanishad with the same name and is contained in the next “three” sections of the Āranyaka viz. from II.4 to II.6. It is here that the following śhlöka explains the entry of the ‘drop’ or ‘spark’ of the Unknown into the head of the new born fetus:-
Ait. Ar. II.4.3 & Ait. Up. I.3.12 [27]
sa etam eva sīmānaṁ vidāryaitayā dvārā prāpadyata, sa eṣā vidṛtir nāma dvāḥ, tad etan nāndanam ; tasya traya āvasathās trayāḥ svapnāḥ,
ayam āvasatho’yam āvasatho’yam āvasatha iti (12)
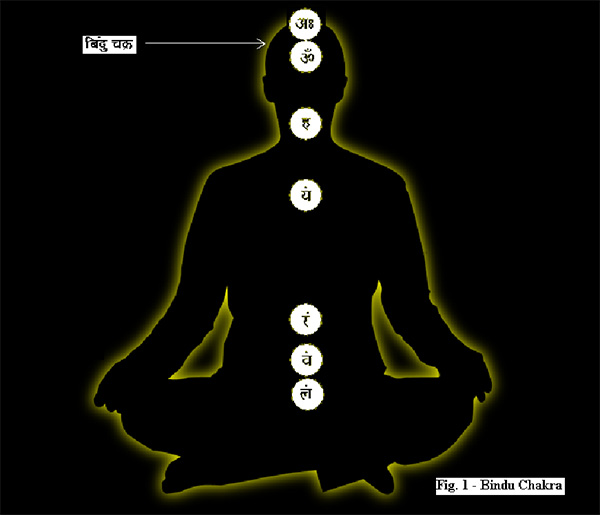
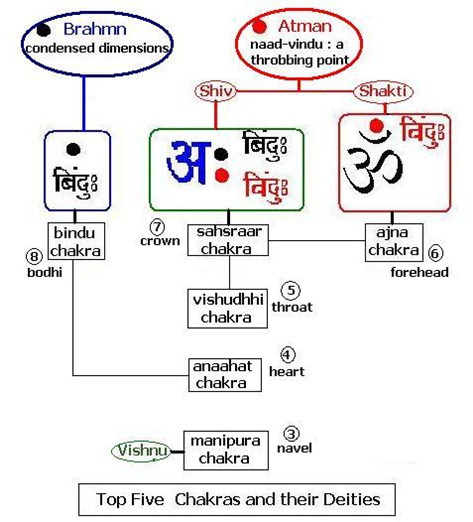
After opening the very end of the head (simānam) , by that way he entered. This is the opening known as (vidriti) . This is the pleasing (naandanam). For that there are three abodes; three kinds of dreams as: this is the abode; this is the abode; this is the abode.[28]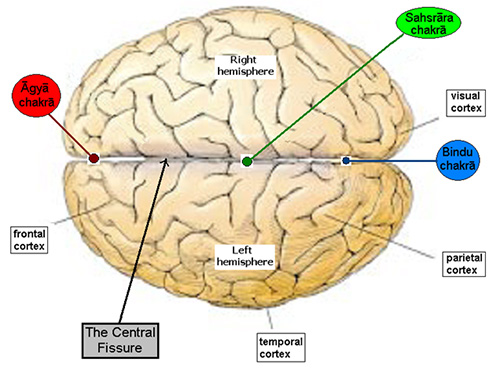 Sīmānam comes from the noun sīme which means boundary or the parting of the hair. Vidriti means the central fissure between the two hemispheres of the brain. See the attached figure that shows the top view of the brain. As we can see this ‘central fissure’ is the abode of ‘three’ chakras viz. the bindu chakra, which is the eighth (this is often overlooked in the popular books on meditation and yoga – it is the ‘hidden’ one)[29], sahasrār chakra – the seventh and the ājna chakra – the sixth.
Sīmānam comes from the noun sīme which means boundary or the parting of the hair. Vidriti means the central fissure between the two hemispheres of the brain. See the attached figure that shows the top view of the brain. As we can see this ‘central fissure’ is the abode of ‘three’ chakras viz. the bindu chakra, which is the eighth (this is often overlooked in the popular books on meditation and yoga – it is the ‘hidden’ one)[29], sahasrār chakra – the seventh and the ājna chakra – the sixth.
- The bindu chakra
It is the point at which the vedic priest or the Brāhman grows a long tuft of hair called the bodhī. The tree by this name is ficus religiosa which is unique in that it has aerial roots that can absorb water from the air and survive in the harshest of climes. One often sees these in India growing in wall crevices or desolate fields. Like the Banyan, ficus bengalensis, the aerial roots of the bodhi tree fall to the ground and keep growing into an ever-expanding umbrella. Buddha found his enlightenment under a bodhi tree. This eighth chakra is also called the bodhi chakra. Each chakra has an inherent, resonant vibration called the beej mantra or literally ‘seed-sound’. The beej of the bodhi chakra is condensed, ‘unheard’ sound. It is a bindu which is often translated as a ‘point’ and, as we will see, it is much more than that. Euclid in his axioms says that – “A point is that which has no part”. In the Vedic context bindu is not inanimate nor is it a simple sound. It is the sum total of all dimensions rolled into a vibrant spot, it is the spanda of the Shiv sūtras [30].
To elaborate, in the eastern context, a ‘point’ cannot be ‘zero-dimensional’. On the contrary, it is a concentrated microcosmic unit that unfolds to reveal all dimensions. It is like the enfolded string of the Unified field theory of physics today. Kalātattvakośa [31] elaborates on bindu giving citations from various Sanskrit texts as follows :-
According to modern geometry the point is the minutest unit with which a line is drawn. The point is indivisible and without length and breadth. When we think of bindu as the minutest unity we are reminded of the concept of paramānu ( Vaiśesika defines paramānu as : mūrtatve sati niravayavah : being limited, it is without any body part ).
In Yogabhāsya of Vyāsa we find that a substance when reduced to its minutest unit is called paramānu , and in the same way the minutest time unit is called ksana. But bindu is neither a time unit like ksana nor a space unit like anu. It is a unit of consciousness , and at the same time becomes body of the material world.
It reminds me of T. S. Eliots’ poem ‘Four Quartets’ in which he refers to a ‘still point’ that is ‘more than a fixity’. I quote from the First Quartet called ‘Burnt Norton’ :-
“At the still point of the turning world. Neither flesh nor fleshless;
Neither from nor towards; at the still point, there the dance is,
But neither arrest nor movement. And do not call it fixity,
Where past and future are gathered. Neither movement from nor towards,
Neither ascent nor decline. Except for the point, the still point,
There would be no dance, and there is only the dance.”
In essence this bindu is a drop of incessant energy which has enfolded vibration but it is ‘non-sound’ or anāhat. That is also the name of the fourth, the heart-chakra. It resonates to a Universal Hum and in Aitareya Āranyaka I.3.1 while explaining the beginning of the offspring from ‘hmm’ , the rishī says “the word is masculine and its cadence feminine…. Again with regard to his (the offspring’s) beginning with the word ‘hmm’, the word is the discrimination of divine and human speech”. This should be understood as the first spurt of soundless prāna from the bodhi chakra to the anāhat or heart chakra inciting the first heartbeat in the womb, the first breath. Thus, in the vedic sense, the seat of cidākāśa, the infinite dimension of undivided consciousness is the hrdya or the heart, although, the antenna is the bindu chakra. The breath or vāyu rises from the lower chakrās to the throat, the seat of the fifth chakra – the viśuddhi chakra, to merge with the prāna from the heartbeat. This first cry is the pranav and is often used synonymously with the sound of ‘aum’ in vedic literature. However, they are different, the former is symbolic of evolution and the latter of the involution of prānic energy. When the conch shell is blown in vedic rituals it is this pranav that is magnified and when we chant inwardly it is the nasal sound of ‘aum’ that resonates in our cranial cavity.
- The sahasrār chakra
This sits as an energy point in the central fissure of the brain under the crown of the head. It is often referred to as the crown chakra.[32] Just as the bodhi chakra is linked to the anāhat or heart chakra, the sahasrār chakra is connected with the viśuddhi or the throat chakra. According to the Vedas, which originated completely as an oral tradition, speech or vāk plays the most important role in identification, naming, recognizing and thus the memorizing of patterns. The entire knowledge of the universe has a nām, name and rūpa or form. The throat is not only resident to speech or vāk it also lends itself to melody or dhvani and the entire gamut of sound is therefore vāni.[33]
The Sanskrit shlökas unfold the sound energies as we view outwards from the Earth and talk to each other. This view is termed bhūgolik or earth-centric and our speech is called vaak. If we look from the heavens downwards, filtering the cacophony, we hear the natural sounds or what is called dhvani or music. And this view is termed khagolik. Thus vaani is the ‘words that are sung’ and it is both vaak and dhvani put together. It is the Divine song.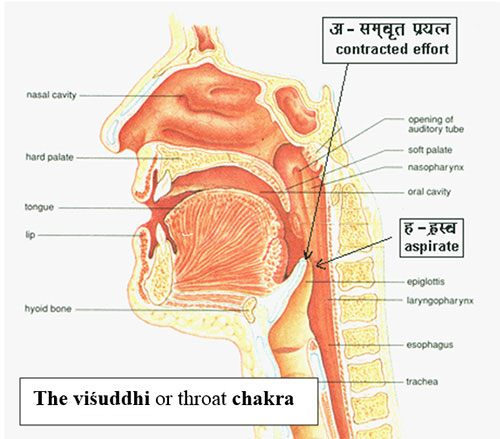 The throat chakra, therefore, is instrumental in harnessing the vāni and through it, the names & forms of the entire creation around us. Whereas the ājna chakra receives and processes the sensory inputs, being surrogate to the cittākāśa and the sahasrār chakra, at the head of the crown, the throat chakra delivers the Ātmic energy into words and music. The beej mantra of the sahasrār chakra is the sound of the first vowel ‘a’ in Sanskrit, combined with ‘h’, the last letter in the Sanskrit alphabet. Interestingly, both these sounds emanate adjacently from the gullet or the kanth. (See attached Figure). This is more explicitly explained by Ait.Ᾱr.II.3.6 :-
The throat chakra, therefore, is instrumental in harnessing the vāni and through it, the names & forms of the entire creation around us. Whereas the ājna chakra receives and processes the sensory inputs, being surrogate to the cittākāśa and the sahasrār chakra, at the head of the crown, the throat chakra delivers the Ātmic energy into words and music. The beej mantra of the sahasrār chakra is the sound of the first vowel ‘a’ in Sanskrit, combined with ‘h’, the last letter in the Sanskrit alphabet. Interestingly, both these sounds emanate adjacently from the gullet or the kanth. (See attached Figure). This is more explicitly explained by Ait.Ᾱr.II.3.6 :-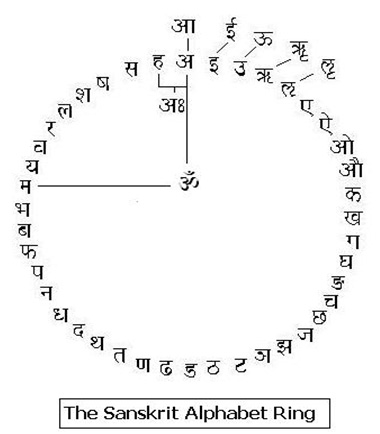 ‘â’ therefore represents ‘that’ which is the Absolute infinity or Omega, Ω of Cantor’s theory of Infinities.[34] It is Brahman, the Unknown. It is the akśara the indestructible or the â + swar, the first vowel. [35]If, this is the ‘whisper’ –as explained in Ait. Ār. II.3.6 above, then as we force the breath just a little, what we get is its aspirate sound ‘ah’. This is the visarga sound, and is grouped as ‘a-yogavāha’ meaning : “those (sounds) that occur (in the actual language) without being part of the alphabet”…another very interesting aspect of the structure of Sanskrit language! If we see the Figure above – another level of symmetry emerges – ‘ah’ closes the circle pictorially signifying the internal – external division . The ‘individual ego’, the ahamkār has taken form – there is an inside and there is an outside – with the language of consciousness as the dividing membrane !
‘â’ therefore represents ‘that’ which is the Absolute infinity or Omega, Ω of Cantor’s theory of Infinities.[34] It is Brahman, the Unknown. It is the akśara the indestructible or the â + swar, the first vowel. [35]If, this is the ‘whisper’ –as explained in Ait. Ār. II.3.6 above, then as we force the breath just a little, what we get is its aspirate sound ‘ah’. This is the visarga sound, and is grouped as ‘a-yogavāha’ meaning : “those (sounds) that occur (in the actual language) without being part of the alphabet”…another very interesting aspect of the structure of Sanskrit language! If we see the Figure above – another level of symmetry emerges – ‘ah’ closes the circle pictorially signifying the internal – external division . The ‘individual ego’, the ahamkār has taken form – there is an inside and there is an outside – with the language of consciousness as the dividing membrane !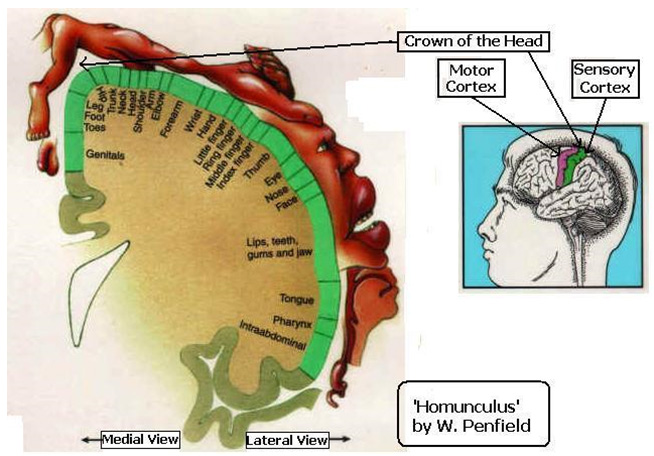 This coincides with Wilder Penfields’ [36] research on the ‘homunculus’ or a little man that he found is located on the cortex of the brain hemispheres on both sides. He was studying the sensory nerves on the skin and found a one-one correspondence for the entire body with this ‘homunculus’ that hangs into the central fissure at the crown of the head – precisely where the sahasrār chakra energizes. As we will see, the bhūtākāśa is centered at the navel or the maṇipūra chakra and gives birth to the physical body. The functions of the brain, its autonomous sensibilities, the ‘awareness’ of the self etc. emanate from the cittākāśa. The self is the ahaṁ; the nasal (anunāsik) sound is added to the ‘ah’. And this nasal resonance at the end of ‘aum’, the universal mantra, is the beej sound for the ājna chakra.
This coincides with Wilder Penfields’ [36] research on the ‘homunculus’ or a little man that he found is located on the cortex of the brain hemispheres on both sides. He was studying the sensory nerves on the skin and found a one-one correspondence for the entire body with this ‘homunculus’ that hangs into the central fissure at the crown of the head – precisely where the sahasrār chakra energizes. As we will see, the bhūtākāśa is centered at the navel or the maṇipūra chakra and gives birth to the physical body. The functions of the brain, its autonomous sensibilities, the ‘awareness’ of the self etc. emanate from the cittākāśa. The self is the ahaṁ; the nasal (anunāsik) sound is added to the ‘ah’. And this nasal resonance at the end of ‘aum’, the universal mantra, is the beej sound for the ājna chakra.
- The ājna chakra
 The ājna chakra is located in the centre of the forehead, exactly where the traditional tikā is to be placed in the Hindu religious practices. This is the seat of the cittākāśa and is denoted by the chandra-vindu that signifies the nasal sound in the symbol of auṁ – the beej mantra for this chakra. This is the 6th chakra and as the learning process of the mind begins in childhood the multidimensional external reality reflects into holographic inputs through the senses [37]. This breaks up the ‘outside universe’ into the various levels of consciousness and the process of assimilation begins in the brain. The ‘mind’ takes shape. The binduh of the bodhi chakra is ‘condensed dimensions’ and the ‘bâ’ sound in binduh is ‘labial plosive’ i.e. it is pronounced by pursing the lips and forcing the breath outwards. The ‘va’ of vinduh of the ājna chakra on the other hand is a ‘labio dental’ belonging to the class of antahsthah or semi-vowels and is pronounced by cutting the lower lip with the upper teeth and pushing the breath inwards and upwards. This is the nād-vinduh of the Shiv sūtras [38]– “the pulsation of the unstruck sound”. Actually, nād itself is not just sound but the entire aspect of semiotic expression including dance, drama etc. [39] Thus binduh signifies an evolution of dimensional energies, whereas, vinduḥ is the involution of external energies.
The ājna chakra is located in the centre of the forehead, exactly where the traditional tikā is to be placed in the Hindu religious practices. This is the seat of the cittākāśa and is denoted by the chandra-vindu that signifies the nasal sound in the symbol of auṁ – the beej mantra for this chakra. This is the 6th chakra and as the learning process of the mind begins in childhood the multidimensional external reality reflects into holographic inputs through the senses [37]. This breaks up the ‘outside universe’ into the various levels of consciousness and the process of assimilation begins in the brain. The ‘mind’ takes shape. The binduh of the bodhi chakra is ‘condensed dimensions’ and the ‘bâ’ sound in binduh is ‘labial plosive’ i.e. it is pronounced by pursing the lips and forcing the breath outwards. The ‘va’ of vinduh of the ājna chakra on the other hand is a ‘labio dental’ belonging to the class of antahsthah or semi-vowels and is pronounced by cutting the lower lip with the upper teeth and pushing the breath inwards and upwards. This is the nād-vinduh of the Shiv sūtras [38]– “the pulsation of the unstruck sound”. Actually, nād itself is not just sound but the entire aspect of semiotic expression including dance, drama etc. [39] Thus binduh signifies an evolution of dimensional energies, whereas, vinduḥ is the involution of external energies.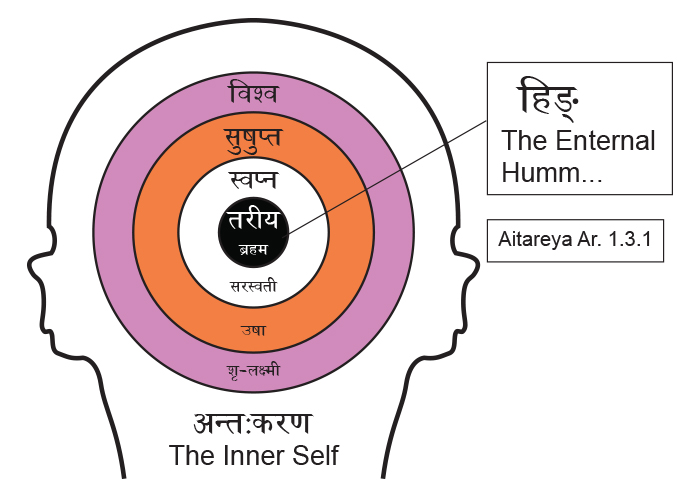 Whereas ‘ah’ stands for the entire alphabet in a circle, aum can be understood as the pratyāhāra for all the vowels plus the mute-consonant sounds. Thus the sound of auṁ traces out the vocal apparatus starting from the glottis to the lips viz. ‘a’ to ‘mâ’. Symbolically, the sibilants or the śhakti letters (sh, shh, sa, ha) and the semi-vowels or the antaḥsthaḥ letters (ya, ra, la, va) are not part of auṁ. This nād-vinduh enfolds the three śhaktis (sh, shh, sa) as the three levels of consciousness given in the Mandukya Upanishad [40] viśwa, suṣhupta and swapna; the fourth sibilant is the aspirate ‘ha’ and it is the enfolded visarga ‘ah’ sound. The fourth level is turīya, the final abode, and it literally means “the fourth”. The attached figure shows these vedic layers of consciousness. The antahsthahs (ya, ra, la, va) are also called the yam symbols and they frame the material support of the body. They are the beej sounds of the 4th, 3rd,1st & 2nd chakras respectively. If we go one step further, we observe, that in the vedic sense mind and body symbols added together complete the cittākāśa or the chaitenya the Consciousness. In other words the Vedas treat the mana or the mind, its inner divisions – the shaktis and the body as one unit. There is no room for mind – body dualism of Descartes. The cittākāśa includes the antahkrana and the bhūtākāśa. There is a clear distinction between the Individual consciousness, the chitt, and the Collective human consciousness, the chaitanya. The former is subdivided into four layers [41] viz. mana or mind, buddhi or intellect / id, ahamkār or ego and ‘ah’ or super-ego. The chaitenya is given in the figure.
Whereas ‘ah’ stands for the entire alphabet in a circle, aum can be understood as the pratyāhāra for all the vowels plus the mute-consonant sounds. Thus the sound of auṁ traces out the vocal apparatus starting from the glottis to the lips viz. ‘a’ to ‘mâ’. Symbolically, the sibilants or the śhakti letters (sh, shh, sa, ha) and the semi-vowels or the antaḥsthaḥ letters (ya, ra, la, va) are not part of auṁ. This nād-vinduh enfolds the three śhaktis (sh, shh, sa) as the three levels of consciousness given in the Mandukya Upanishad [40] viśwa, suṣhupta and swapna; the fourth sibilant is the aspirate ‘ha’ and it is the enfolded visarga ‘ah’ sound. The fourth level is turīya, the final abode, and it literally means “the fourth”. The attached figure shows these vedic layers of consciousness. The antahsthahs (ya, ra, la, va) are also called the yam symbols and they frame the material support of the body. They are the beej sounds of the 4th, 3rd,1st & 2nd chakras respectively. If we go one step further, we observe, that in the vedic sense mind and body symbols added together complete the cittākāśa or the chaitenya the Consciousness. In other words the Vedas treat the mana or the mind, its inner divisions – the shaktis and the body as one unit. There is no room for mind – body dualism of Descartes. The cittākāśa includes the antahkrana and the bhūtākāśa. There is a clear distinction between the Individual consciousness, the chitt, and the Collective human consciousness, the chaitanya. The former is subdivided into four layers [41] viz. mana or mind, buddhi or intellect / id, ahamkār or ego and ‘ah’ or super-ego. The chaitenya is given in the figure. - The maṇipūra chakra
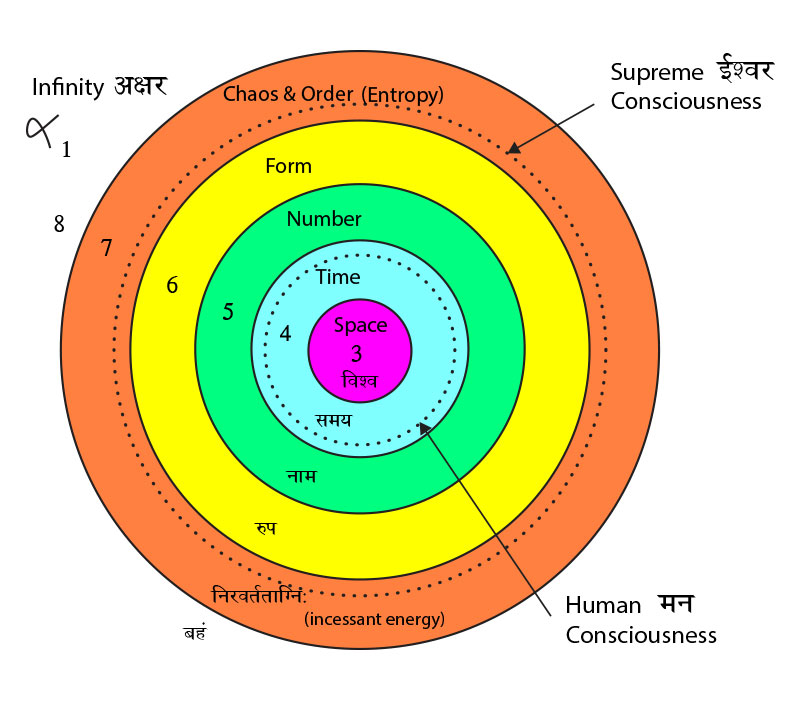
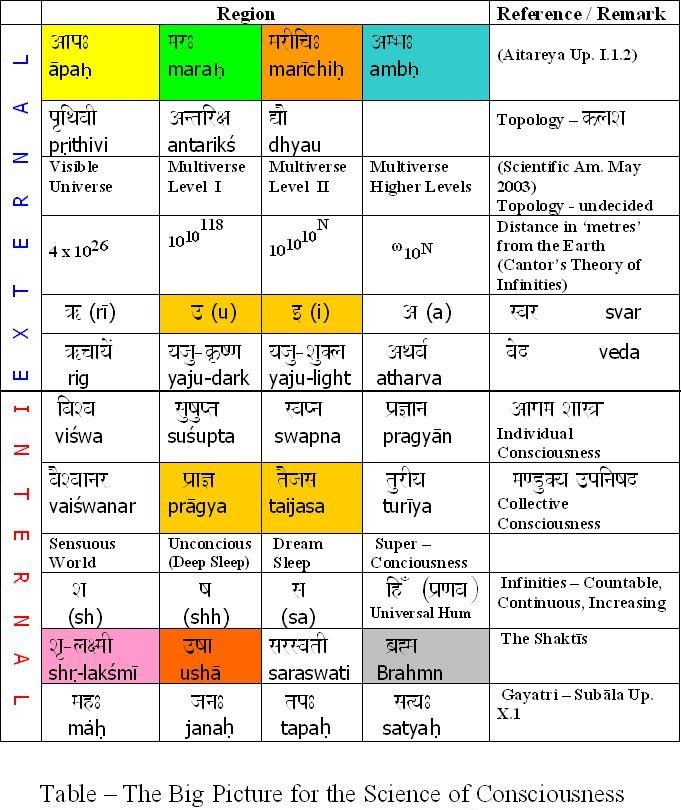
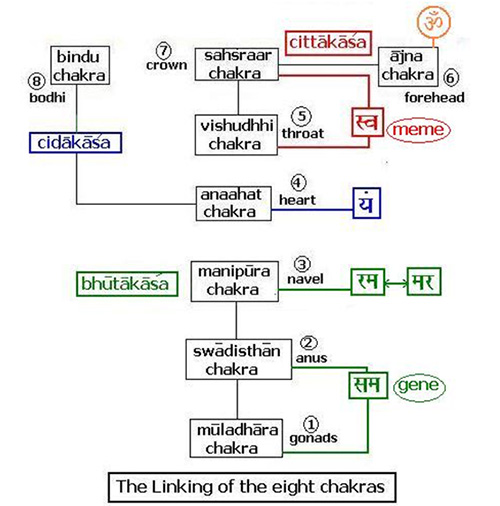 Situated at the navel or the nābhi this chakra is the life support point of the human body. This is where the umbilical cord connects to the unborn child and this is the center of the forming foetus. The bhūtākāśa is constituted of the lowest three chakras – the mūladhāra, the swādiśṭhān and the maṇipūra. The first letters of the swādiśṭhān and the mūladhāra give us the clue to its continuity – they spell sam which is the prefix meaning ‘uniformity’. This is what makes samsāra a ‘uniform verse’ – the universe. For, in the vedic tradition the entire creation has emerged from complete order and an Unknown perfection – a ‘uniformity’, the Brahmn. This timeless sam of order is the gene. Similarly, the acronym for the sahasrār and viśuddhi chakra is sva which means the self. Just as the gene maintains its order and carries the stages of the evolution of life within it, similarly, the sva or the ‘meme’ [42] carries with it the body of knowledge from memory to memory i.e. generations to generations. This was the oral tradition of the transmission of the knowledge of the Vedas.
Situated at the navel or the nābhi this chakra is the life support point of the human body. This is where the umbilical cord connects to the unborn child and this is the center of the forming foetus. The bhūtākāśa is constituted of the lowest three chakras – the mūladhāra, the swādiśṭhān and the maṇipūra. The first letters of the swādiśṭhān and the mūladhāra give us the clue to its continuity – they spell sam which is the prefix meaning ‘uniformity’. This is what makes samsāra a ‘uniform verse’ – the universe. For, in the vedic tradition the entire creation has emerged from complete order and an Unknown perfection – a ‘uniformity’, the Brahmn. This timeless sam of order is the gene. Similarly, the acronym for the sahasrār and viśuddhi chakra is sva which means the self. Just as the gene maintains its order and carries the stages of the evolution of life within it, similarly, the sva or the ‘meme’ [42] carries with it the body of knowledge from memory to memory i.e. generations to generations. This was the oral tradition of the transmission of the knowledge of the Vedas.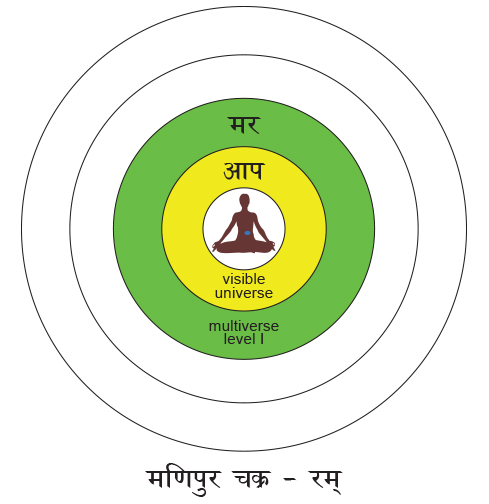 The beej mantra for the manipūra chakra is ‘raṁ’ which means ‘complete absorption of thought’ and in fact the mythological name Rāma means ‘the one who is so perfect that he absorbs your entire being’. The higher dimensions in terms of the Vedas are beyond time and space and the seeds of higher consciousness are present as ‘symmetries’ in our limited experience. Life and the mind are inextricably interlinked and the symmetries are ensconced one within the other like ‘the endless dance of Chinese dolls’. You remove one symmetry and a smaller one appears. This universe is non-linear and our sensuous limitations make the unfolding dimensions linear.[43] The womb protects the cell multiplication of the ‘conceptus’ [44] and the collective symmetries of the entire emergence of life on earth come to play. I quote from [45]:-The Nirukta (2.8)[46] first relates the root √ma to mātā which literally means “the atmosphere” encircling the earth. This also means the “mother’s womb”; in it the ātmā, the soul takes shape and form and is born in this world. Just like the womb encloses the child and protects it, similarly the atmosphere covers the earth. Note that māta – ātmā are conjugal words.The next level of symmetry is the ‘visible universe’ or āpo. The visibility is due to the dispersal of energy from the time of the ‘Big Bang’ that has ultimately germinated the cellular revolution on this uniquely placed planet. The region that is visible is therefore termed as vyāpak [47]. The region beyond this is called mar which literally means ‘death’. The latter is the realm of the ‘dark matter’. It is interstitial with the ‘shining galaxies’ and cannot be seen because it absorbs light instead of emanating it. The two together have constituted all matter that supports our being and continuously flows through us – changing every atom within our body completely every seven years or so. This is the limit of the bhūtākāśa that has constituted the very structure of our body and brain. The primary consciousness rests on our ‘looking back’ at the vyāpak universe and it unfolds the layer of viśwa in the antahkrana or the ‘inner self’ as we accumulate knowledge through our senses. The autonomous nervous system is related to the subconscious mind that controls the basic bodily functions. This is resident in the inner cortices of the brain that have developed in the first animals and frames the ‘archicortex’ and the ‘mesocortex’ of the mammalian brain.[48] This subconscious layer gives rise to the sushupta. In the Vedic sense the souls that do not evolve in their daily lives and are immersed in these two levels, go back and forth between the mar – āpo region in an endless cycle of reincarnation. The ‘neocortex’ gives the ability to the mammalian brain to evolve to even higher forms and thus connect with regions of the multiverse beyond our ken.
The beej mantra for the manipūra chakra is ‘raṁ’ which means ‘complete absorption of thought’ and in fact the mythological name Rāma means ‘the one who is so perfect that he absorbs your entire being’. The higher dimensions in terms of the Vedas are beyond time and space and the seeds of higher consciousness are present as ‘symmetries’ in our limited experience. Life and the mind are inextricably interlinked and the symmetries are ensconced one within the other like ‘the endless dance of Chinese dolls’. You remove one symmetry and a smaller one appears. This universe is non-linear and our sensuous limitations make the unfolding dimensions linear.[43] The womb protects the cell multiplication of the ‘conceptus’ [44] and the collective symmetries of the entire emergence of life on earth come to play. I quote from [45]:-The Nirukta (2.8)[46] first relates the root √ma to mātā which literally means “the atmosphere” encircling the earth. This also means the “mother’s womb”; in it the ātmā, the soul takes shape and form and is born in this world. Just like the womb encloses the child and protects it, similarly the atmosphere covers the earth. Note that māta – ātmā are conjugal words.The next level of symmetry is the ‘visible universe’ or āpo. The visibility is due to the dispersal of energy from the time of the ‘Big Bang’ that has ultimately germinated the cellular revolution on this uniquely placed planet. The region that is visible is therefore termed as vyāpak [47]. The region beyond this is called mar which literally means ‘death’. The latter is the realm of the ‘dark matter’. It is interstitial with the ‘shining galaxies’ and cannot be seen because it absorbs light instead of emanating it. The two together have constituted all matter that supports our being and continuously flows through us – changing every atom within our body completely every seven years or so. This is the limit of the bhūtākāśa that has constituted the very structure of our body and brain. The primary consciousness rests on our ‘looking back’ at the vyāpak universe and it unfolds the layer of viśwa in the antahkrana or the ‘inner self’ as we accumulate knowledge through our senses. The autonomous nervous system is related to the subconscious mind that controls the basic bodily functions. This is resident in the inner cortices of the brain that have developed in the first animals and frames the ‘archicortex’ and the ‘mesocortex’ of the mammalian brain.[48] This subconscious layer gives rise to the sushupta. In the Vedic sense the souls that do not evolve in their daily lives and are immersed in these two levels, go back and forth between the mar – āpo region in an endless cycle of reincarnation. The ‘neocortex’ gives the ability to the mammalian brain to evolve to even higher forms and thus connect with regions of the multiverse beyond our ken. - The Vedic Multiverse The Aitareya Āranyaka II.4.2 or the Aitareya Upaniṣad I.1.2 explains this and more: sa imān lokān asrjat ; ambho, marīchīh, maram, āpo ; ado ambh paren divam dhyau pratisṭhā antarikśammarīchyah prthivī maro ya adhastāt tā āpah (2)
-
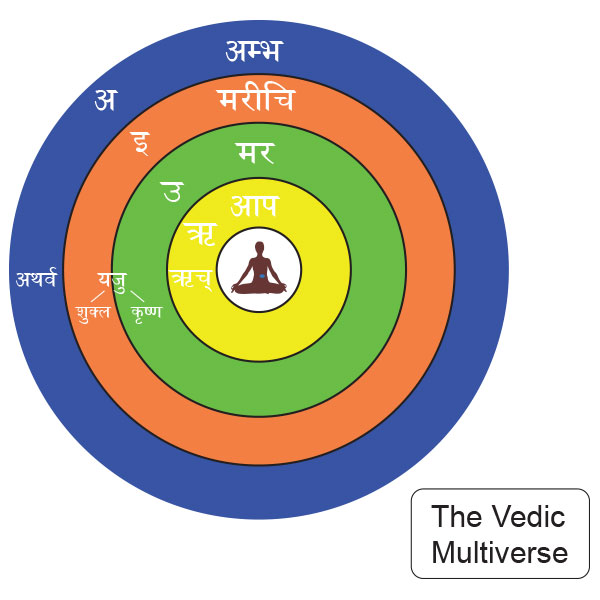 This particular shlöka cannot be translated simply and has to be understood pictorially. The region that is beyond the mar is marīchi and this is the circle of light again. This is the dev-lök alluded to in many a myth. The access to this is through intent that creates a desire and its ‘need to happen’ dichotomy in the mind. This is the saṁkalpa – vikalpa of the Upanishads and occurs in the swapna layer of the consciousness. The latent desires of the mind are probabilistic in occurrence – they may or may not happen in the larger world. However, in the micro-world of Quantum Mechanics (QM) extremely rapid fluctuations of matter precipitate these probabilities almost instantaneously because a very large number of ‘particles’ are involved in all processes. The entire material reality is based on these QM uncertainties and many a popular text has been written on the wave-particle duality[49] and Heisenberg’s Uncertainty Principle. In mathematical terms, in QM, all possibilities can be said to be present simultaneously as a ‘probabilistic wave field’ and the one chance that precipitates action causes an ‘instantaneous collapse of this field’. This has created huge conceptual and philosophical ‘waves’ of their own in the field of Physics and to address this problem Hugh Everett III in 1957, a 19 year old graduate student of Princeton University, came up with “The Many World Theory”. This has been recently elaborated by Max Tegmark[50] in 2003 and also presented by Time Magazine in a Dec 2004 article. The concept is now called “Parallel Universes”. In his introduction of the Scientific American article Tegmark says:-
This particular shlöka cannot be translated simply and has to be understood pictorially. The region that is beyond the mar is marīchi and this is the circle of light again. This is the dev-lök alluded to in many a myth. The access to this is through intent that creates a desire and its ‘need to happen’ dichotomy in the mind. This is the saṁkalpa – vikalpa of the Upanishads and occurs in the swapna layer of the consciousness. The latent desires of the mind are probabilistic in occurrence – they may or may not happen in the larger world. However, in the micro-world of Quantum Mechanics (QM) extremely rapid fluctuations of matter precipitate these probabilities almost instantaneously because a very large number of ‘particles’ are involved in all processes. The entire material reality is based on these QM uncertainties and many a popular text has been written on the wave-particle duality[49] and Heisenberg’s Uncertainty Principle. In mathematical terms, in QM, all possibilities can be said to be present simultaneously as a ‘probabilistic wave field’ and the one chance that precipitates action causes an ‘instantaneous collapse of this field’. This has created huge conceptual and philosophical ‘waves’ of their own in the field of Physics and to address this problem Hugh Everett III in 1957, a 19 year old graduate student of Princeton University, came up with “The Many World Theory”. This has been recently elaborated by Max Tegmark[50] in 2003 and also presented by Time Magazine in a Dec 2004 article. The concept is now called “Parallel Universes”. In his introduction of the Scientific American article Tegmark says:- Is there a copy of you reading this article? A person who is not you but lives on a planet Earth, with misty mountains, fertile fields and sprawling cities, in a solar system with eight other planets…..The idea of such an alter ego seems strange and implausible, but it looks as if we will just have to live with it, because it is supported by astronomical observations. The simplest and most popular cosmological model today predicts that you have a twin in a galaxy about 10 to the power of 1028 meters from here. This distance is so large that it is beyond astronomical but that does not make your doppelgänger any less real. The estimate is derived from elementary probability and does not even assume speculative modern physics, merely that space is infinite (or at least sufficiently large) in size and almost uniformly filled with matter, as observations indicate. In infinite space, even the most unlikely events must take place somewhere. There are infinitely many other inhabited planets, including not just one but infinitely many that have people with the same appearance, name and memories as you, who play out every possible permutation of your life choices.The frontiers of physics have gradually expanded to incorporate ever abstract (and once metaphysical) concepts such as a round Earth, invisible electromagnetic fields, time slowdown at high speeds, quantum superposition, curved space, and black holes. Over the past several years the concept of a multiverse has joined this list. It is grounded in well-tested theories such as relativity and quantum mechanics…. Scientists have discussed as many as four distinct types of parallel universes. The key question is not whether the multiverse exists but rather how many levels it has. Motilal Shastri (1908 – 1960), a Vedic scholar of rare insight has written vigyānbhāsyam or scientific treatise on a number of Upanishads and the Śatpath Brāhmana. Explaining the hiranyagarbh or the ‘Golden seed’ of the Universe (Multiverse) he explains the four regions in great detail[51]:-
Is there a copy of you reading this article? A person who is not you but lives on a planet Earth, with misty mountains, fertile fields and sprawling cities, in a solar system with eight other planets…..The idea of such an alter ego seems strange and implausible, but it looks as if we will just have to live with it, because it is supported by astronomical observations. The simplest and most popular cosmological model today predicts that you have a twin in a galaxy about 10 to the power of 1028 meters from here. This distance is so large that it is beyond astronomical but that does not make your doppelgänger any less real. The estimate is derived from elementary probability and does not even assume speculative modern physics, merely that space is infinite (or at least sufficiently large) in size and almost uniformly filled with matter, as observations indicate. In infinite space, even the most unlikely events must take place somewhere. There are infinitely many other inhabited planets, including not just one but infinitely many that have people with the same appearance, name and memories as you, who play out every possible permutation of your life choices.The frontiers of physics have gradually expanded to incorporate ever abstract (and once metaphysical) concepts such as a round Earth, invisible electromagnetic fields, time slowdown at high speeds, quantum superposition, curved space, and black holes. Over the past several years the concept of a multiverse has joined this list. It is grounded in well-tested theories such as relativity and quantum mechanics…. Scientists have discussed as many as four distinct types of parallel universes. The key question is not whether the multiverse exists but rather how many levels it has. Motilal Shastri (1908 – 1960), a Vedic scholar of rare insight has written vigyānbhāsyam or scientific treatise on a number of Upanishads and the Śatpath Brāhmana. Explaining the hiranyagarbh or the ‘Golden seed’ of the Universe (Multiverse) he explains the four regions in great detail[51]:-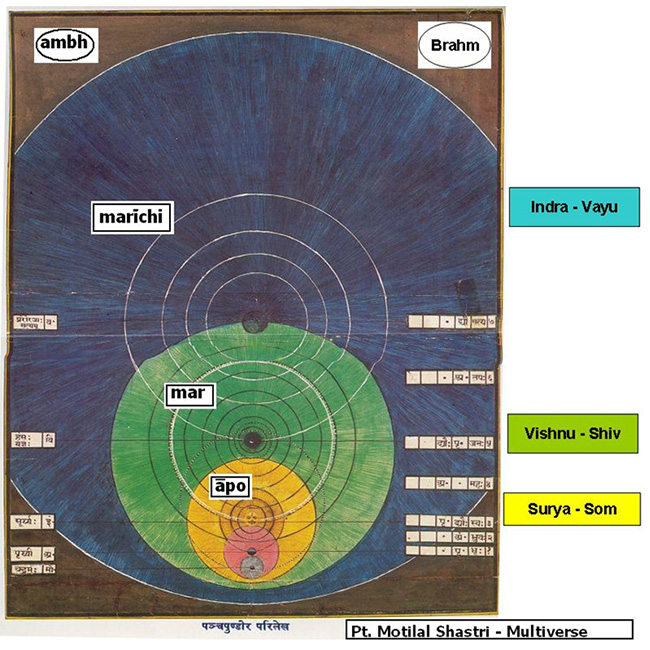 First we must understand that the terms prthivī, antarikś and dhyauh are generic. The first comes from the root √prath which means a level region. The word prthivī therefore connotes ‘an extremely large sphere that locally gives the impression of flatness’. The second region, antarikś comes from the word antara that means both ‘in between’ two regions and ‘interior space’. The third, dhyauh comes from the root √div which means ‘expansive radiation’ and is often translated as the ‘light of knowledge’. Each of the regions has this trio and the dhyauh of the āpo is the prthivī of the next region i.e. mar and so on and so forth). The āpo region has the Earth as pṛthivī and the antarikś is the inter galactic space. The dhyauh is the limit of the visible universe and it is the Hubble volume of the Multiverse theory. The play of energy here is between the sūrya, the sun and som, the moon. These are the tides that directly play on the creation at this level. The gāyatri vyāhrtyah are seven in number and the pṛthivī here is the bhuḥ, the antarikś is the bhuvaḥ and the dhyauh is the svah). The mar region again starts with pṛthivī, that is the dhyauḥ of the āpo. This is the end of the Hubble sphere and is beyond our senses. This is where the Multiverse Level I of Tegmark begins. The inter-space between them is the antarikś and the dhyauh is the limit of the ‘Our Level I Multiverse’ as shown in the bubble in the picture here.
First we must understand that the terms prthivī, antarikś and dhyauh are generic. The first comes from the root √prath which means a level region. The word prthivī therefore connotes ‘an extremely large sphere that locally gives the impression of flatness’. The second region, antarikś comes from the word antara that means both ‘in between’ two regions and ‘interior space’. The third, dhyauh comes from the root √div which means ‘expansive radiation’ and is often translated as the ‘light of knowledge’. Each of the regions has this trio and the dhyauh of the āpo is the prthivī of the next region i.e. mar and so on and so forth). The āpo region has the Earth as pṛthivī and the antarikś is the inter galactic space. The dhyauh is the limit of the visible universe and it is the Hubble volume of the Multiverse theory. The play of energy here is between the sūrya, the sun and som, the moon. These are the tides that directly play on the creation at this level. The gāyatri vyāhrtyah are seven in number and the pṛthivī here is the bhuḥ, the antarikś is the bhuvaḥ and the dhyauh is the svah). The mar region again starts with pṛthivī, that is the dhyauḥ of the āpo. This is the end of the Hubble sphere and is beyond our senses. This is where the Multiverse Level I of Tegmark begins. The inter-space between them is the antarikś and the dhyauh is the limit of the ‘Our Level I Multiverse’ as shown in the bubble in the picture here. 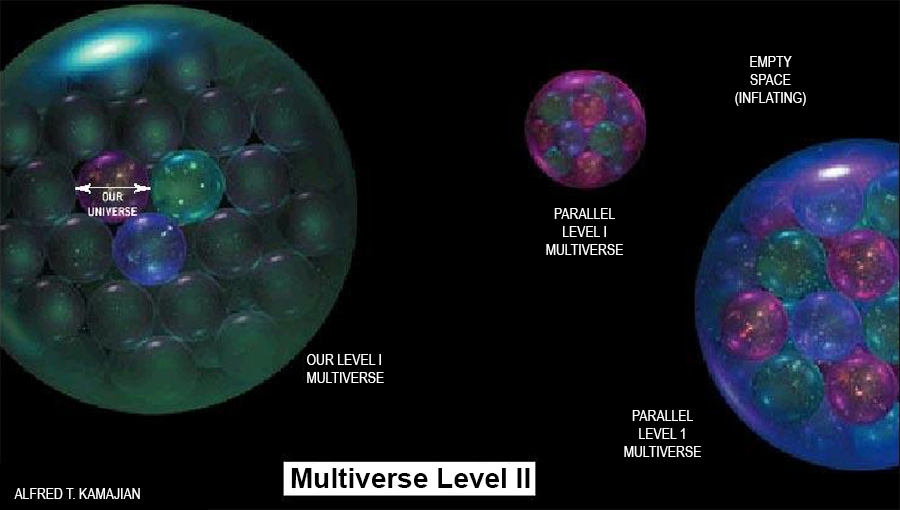 This then becomes the prthivī for the next region marīchi. The energy in mar springs between Viṣṇu and Shiv. Note that at the external level this is the limit of the bhūtākāśa and there too the ruling deity is Viṣṇu. At the internal level Shiv also rules the sva of the cittākāśa along with śakti. In Hindu mythology Shiv is the chaotic destroyer and Viṣṇu is the one that preserves order. In the Multiverse, above the visible universe, tremendous energies are released by the ‘big bangs’ of each Hubble volume, and their black holes are coalescing the multiple galaxies back under crushing gravitational forces. The expanding energies are Viṣṇu that are always depicted with agni or fire emanating from his head. The root verb ‘ag’ means ‘a tortuous movement’ and the suffix ‘ni’ means in this context ‘to throw out’. So, agni is ‘the flickering of the flame’ that shines and spews forth energy. The opposite is n-agni or nagini that means a snake, literally ‘a black tortuous movement’ that absorbs energy. Thus Shiv is portrayed with snakes around his neck.The three gāyatri vyāhrtyaḥ here are svah, maha and janaḥ. Note that the fourth vyāhrtyah , maha is aham backwards and the two are conjugal words.
This then becomes the prthivī for the next region marīchi. The energy in mar springs between Viṣṇu and Shiv. Note that at the external level this is the limit of the bhūtākāśa and there too the ruling deity is Viṣṇu. At the internal level Shiv also rules the sva of the cittākāśa along with śakti. In Hindu mythology Shiv is the chaotic destroyer and Viṣṇu is the one that preserves order. In the Multiverse, above the visible universe, tremendous energies are released by the ‘big bangs’ of each Hubble volume, and their black holes are coalescing the multiple galaxies back under crushing gravitational forces. The expanding energies are Viṣṇu that are always depicted with agni or fire emanating from his head. The root verb ‘ag’ means ‘a tortuous movement’ and the suffix ‘ni’ means in this context ‘to throw out’. So, agni is ‘the flickering of the flame’ that shines and spews forth energy. The opposite is n-agni or nagini that means a snake, literally ‘a black tortuous movement’ that absorbs energy. Thus Shiv is portrayed with snakes around his neck.The three gāyatri vyāhrtyaḥ here are svah, maha and janaḥ. Note that the fourth vyāhrtyah , maha is aham backwards and the two are conjugal words.
c) The marīchi is the Multiverse Level II of Tegmark with the round Multiverse Level I globe as its prthivī, the space between two such globes as its antarikś and again at a higher level a dhyauh. By now the number of possible Earths and our doppelgängers has increased so large that it can take care of all the alternatives that we may take in our limited lifetime! This is the dev lök where Lord Indra holds his sway of merry song and dance in the myths. When you get a knock on the head and see light, you experience a very brief ‘Altered State of Consciousness’ [52] for a split second. If extended in experience it is an ‘insight’: a divyajyoti. At the time of meditation and repetitive chanting – it is this region that comes into play. The ruling energies are Indra and Vāyu. The Aitareya Upaniṣad I.3.14 [53] explains why Indra is so named. Idam means ‘this’ and ‘that which can be seen’ is idam adarśam. This is shortened to idamdra and further to Indra, a cryptic version ‘because the Gods like mysterious names’. In essence Indra is the perceivable aspect of the Unknown, Brahmn. The Vāyu is also an attractive force and in the Aitareya Āranyaka I.1.4.4 the Hotr priest offers honey to the Indra and Vāyu “Now food verily is honey, for all is honey, all desires are honey, and thus if he recites the madhuchhandas, it serves for the attainment of all desires.” [54] Honey is used as a metaphor because its drops coalesce and satiate hunger. This is the essence of this region. The three gāyatri vyāhrtyah here are janah, tapah and satya. The last is the realm of Brahmn, that besides being the Unknown is also the eternal truth, for satya means truth.
So, in the Vedic essence, the spark of the soul survives death and sits in the marīchi – the Multiverse Level II, where all intentions are feasible. A region way beyond our direct senses but yet inextricably linked to our minds through our desires, the swapna. And here the antic waits its turn to re-incarnate…….
The final release can be achieved if we access the turīya in the mind, and this can ‘transport’ the soul to the Unknown region, ambh, ruled by the Brahmn.
THE KNOWLEDGE OF THE VEDAS
Each region has its own body of knowledge and it is called the Veda. The first svar or vowel ‘a’ stands for Atharva Veda that explains the mysteries of the ambh. The second vowel and the third vowel ‘i’ and ‘u’ combine to give Yaju Veda for the ‘ya’ semi-vowel contains the ‘i’ sound. The knowledge of marīchi is in the Yaju – śukla (light) and the mar is expounded upon in the Yaju – krisna (dark). The fourth vowel is ‘ṛ’ (pronounced ‘ri’) and the richa of Rig Veda are replete with the rhythms of the visible universe, āpo. The Sām Veda is to do with sva, the mind and contains that which feeds the inner self.
This then is the big picture of Consciousness. I have tried to trace its scientific basis from matter to mind and looked at the equivalent concepts in the Vedas and the Upanishads. I can see the convergence and the amalgamation of these two lines of intense human endeavor into a new theory of dimensions.
(The table at the end summarizes this talk).